
We will discuss the relationship between intermolecular forces and physical properties later, but for now, keep in mind that the stronger these interactions the higher the melting and boiling points are. In other words, there is not really such thing as one bond between two ions/atoms like in covalent compounds and that’s why they need a lot of thermal energy to break these interactions and therefore, have high melting points. Unlike the covalent bonding, ionic bonds are not directional and specific between given atoms, but rather they form a lattice as an interaction among the entire cluster of the ions:
What is important here is that we are not talking about the interaction of one Na + and Cl + ion together. Among other interesting features and applications, we learn that it has a melting point of 801 ☌ which is extremely higher than the typical range for organic (covalent) compounds. For example, sodium chloride (NaCl) is one of the first ionic compounds we talk about in general chemistry. All of them are electrostatic interactions meaning that they all occur as a result of the attraction between opposite charges and which of these forces is present or predominates in a given compound, depends on its functional groups.īack from general chemistry, we know from the structure of salts that oppositely charged particles tend to show very strong electrostatic interactions and these are the ionic compounds. The reason non-polar substances do not dissolve in water has nothing to do with the energetics of hydrogen bonding.Dipole-dipole, London dispersion (also known as Van der Waals) interactions, hydrogen bonding, and ionic bonds are the main types of intermolecular interactions responsible for the physical properties of compounds. Most people attribute this to the strength of hydrogen bonding, but that turns out to not be the case. A good example of this is the fact that non-polar substances do not dissolve in water. The MCAT often seems to test these topics in the context of solubility, which tends to be governed by the energetics (i.e., enthalpies) of the interactions, although not always.

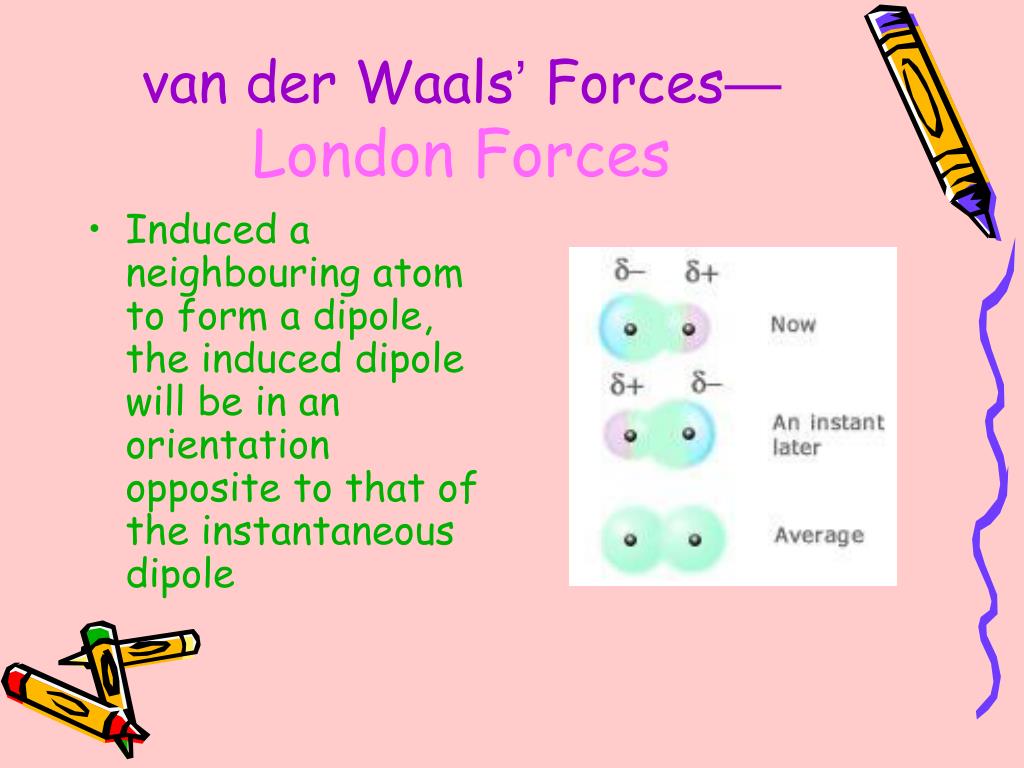
It's far more important that you understand what leads to all of the intermolecular interactions and are able to rank them. This distinction doesn't really get stressed on the MCAT anyway. Technically speaking, London dispersion forces refer strictly to induced-dipole interactions, which are a subset of van der Waal's forces. Others use the term to refer to induced-dipole attraction, which is what seems to be how the MCAT treats it. Some use van der Waal's forces to refer to all of the intermolecular attractive forces except for covalent, ionic, or hydrogen bonds.

The definition of van der Waal's forces is not entirely agreed upon. This is where the MCAT gets a little annoying. An individual hydrogen bond is not especially strong, but when your solvent is capable of accepting and donating hydrogen bonds, the interactions become extremely favorable. What makes hydrogen bonds special is that you can make lots of them. As dipole-dipole interactions go, they're not necessarily the strongest either. Hydrogen bonds are not stronger than dipole-dipole interactions.

This is not entirely accurate and unfortunately, the MCAT does test this pretty heavily, so it's worth your time to get it right.


 0 kommentar(er)
0 kommentar(er)
